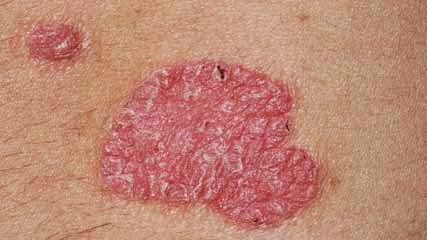
Incidence rates of malignancies and infections in psoriasis
This paper is published in the BJD this month in the epidemiology and health services research section. It looks at comparing groups from the general population, those with psoriasis receiving treatment and those with psoriasis who are not receiving treatment, comparing the incidence of malignancies and hospitalised infectious events in patients in the USA (2005 - 2009). The findings reveal that there are higher rates of both in patients with psoriasis, however it is unclear as to whether these higher rates are associated with an underlying inflammatory state, treatments being given or perhaps both. The authors of the study wrote in their conclusions that the elevated rates of malignancy and serious infections found in patients with psoirasis may be due to biological reasons, since there was little difference in rates between treated and untreated groups. A lot is already known about the risk of cardiovascular disease in patients with psoriasis but this study adds more weight to the evidence of risk of other disease processes such as susceptibility to infections and malignant disease.
Kimball AB et al. Incidence rates of malignancies and hospitalised infectious events in patients with psoriasis with or without treatment and a general population in the USA: 2005-2009. Br J Dermatol 2014; 170:366-373
Efficacy and safety of systemic treatments for moderate to severe psoriasis
This second paper published in the BJD in February is a systematic review of randomised controlled trials comparing the efficacy and safety of biologics used to treat psoriasis. The PASI (psoriasis area and severity index) was used as the primary measure of efficacy (at week 8-16). 48 RCTs were included in the review (total of 16,696 patients) and the conclusions for the authors were that both qualitative and quantitative outcome evidence was much stronger for patients receiving biological, compared with conventional treatments. Unfortunately, safety of treatments could not be pooled and this was due to a lack of standardisation of reporting across the trials. This shows that more work needs to be done to assess the safety of biologics and this can be achieved through a standardisation process for how safety events (rates of adverse events and withdrawals) are reported.
Schmitt J et al. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomised controlled trials. Br J Dermatol 2014; 170:274-304